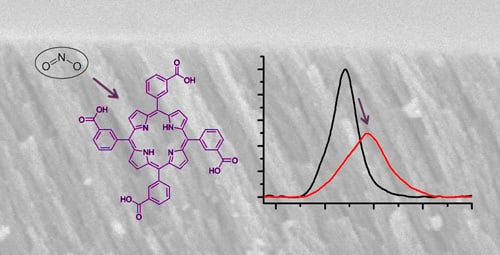
Sensors | Free Full-Text | Free-Base Carboxyphenyl Porphyrin Films Using a TiO2 Columnar Matrix: Characterization and Application as NO2 Sensors

Yoshi Baza hybrydowa do paznokci Rubber Base UV Hybrid No2 10ml Rubber Base No2 | Paznokcie \ Bazy i topy Promocje Promocja - Kosmetyki Plus

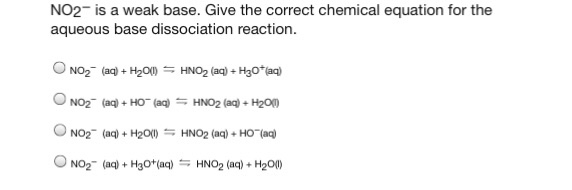
1. What is the Bronsted Acid in the following equation: * NO2- +H2O HNO2 + OH- **a. NO2- **b. H2O **c. HNO2 **d. OH- 2. What is the Bronsted base in the

In this reaction, does NO2+ act like a Lewis acid, Lewis base, Bronsted acid or Bronsted base? A. Lewis Acid B. Lewis Base C. Bronsted Acid D. Bronsted Base E. Lewis Acid
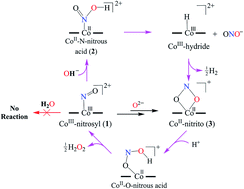
Nitric oxide monooxygenation (NOM) reaction of cobalt-nitrosyl {Co(NO)}8 to CoII-nitrito {CoII(NO2−)}: base induced hydrogen gas (H2) evolution - Chemical Science (RSC Publishing)

organic chemistry - What are the resulting conjugate acid and base of phenol and 4-nitrophenol - Chemistry Stack Exchange

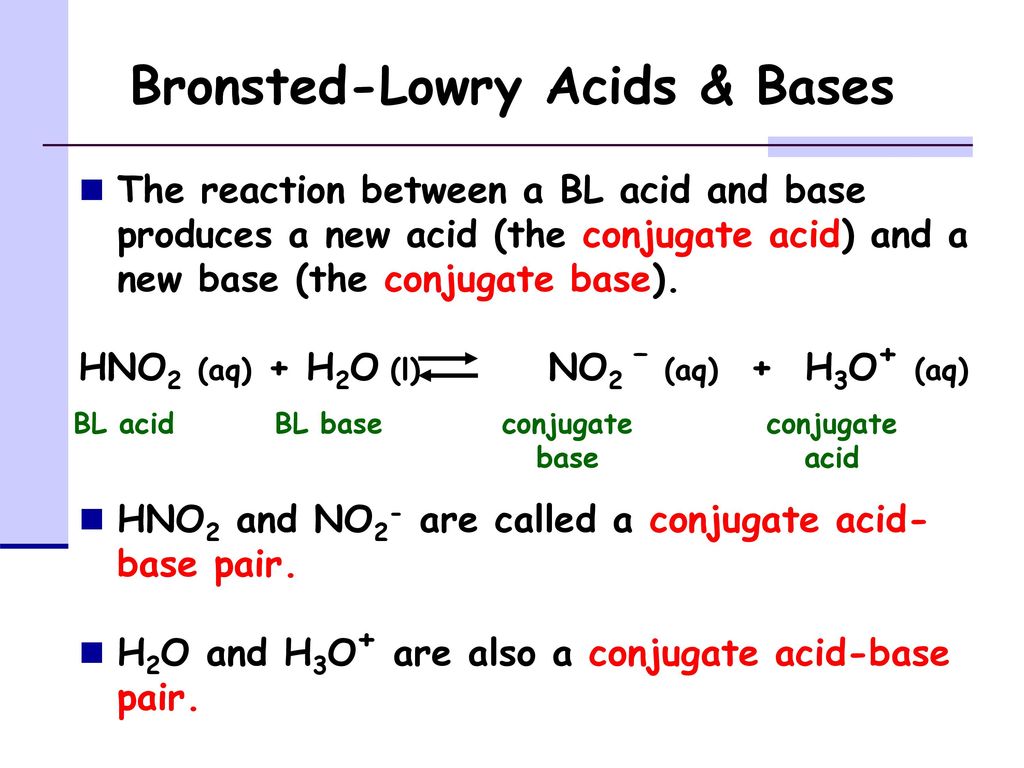
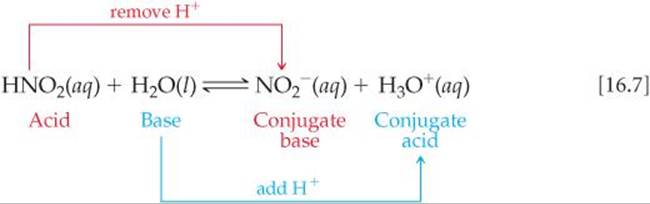
SOLVED: 19. Which of the following is not conjugate acid-base pair? A) Hz0,HO B) H2O, Hzot C) HSO4 , HzS04 D) -OH,02- E) NO3 , NO2"