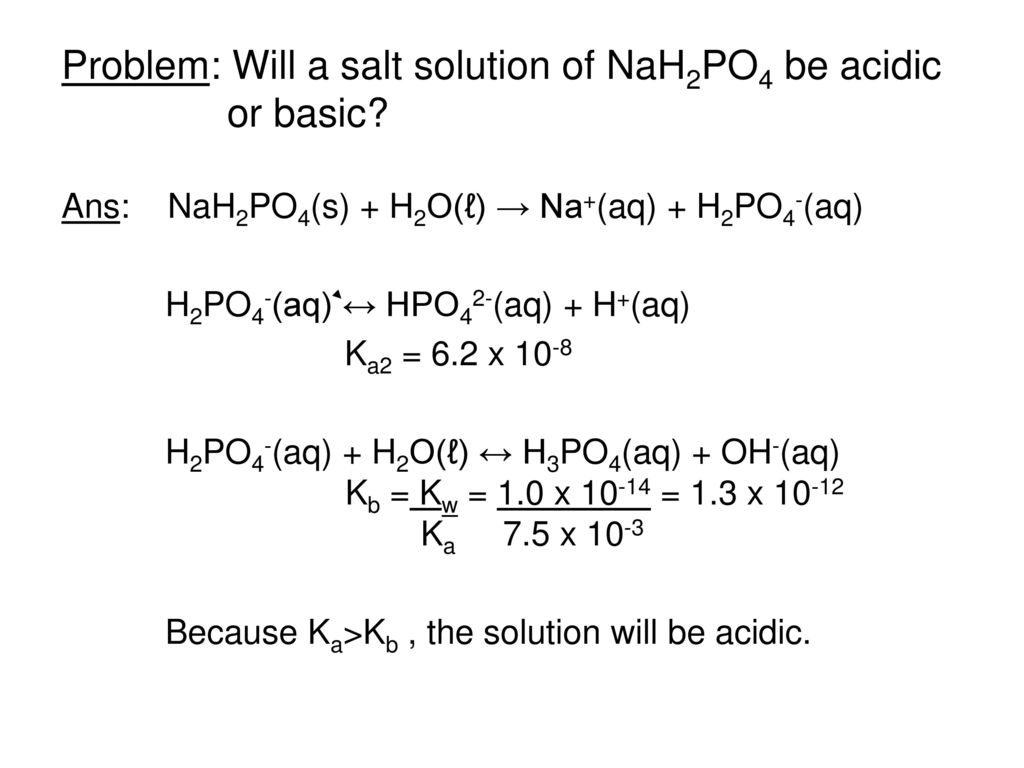
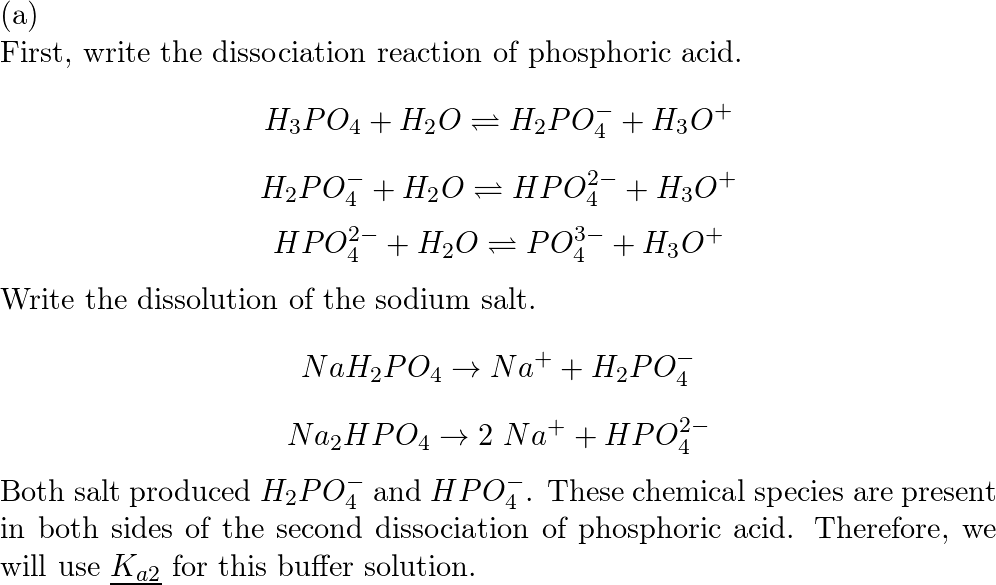Phase Equilibrium for the Ternary System KH2PO4 + NaH2PO4 + H2O at 303.15 K | Journal of Chemical & Engineering Data
![Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/NaH2PO4s%20500G.jpg)
Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs

The equivalent weight of NaH2PO4 in the reaction NaH2PO4+KOH→NaKHPO4 + H2O (Given Atomic masses: Na = 23. K = 39, P = 31)

Valores médios da concentração de H x PO 4 3-x presentes na solução de... | Download Scientific Diagram

1 Bán Hóa chất Sodium dihydrogen phosphate monohydrat, reagent grade - NaH2PO4.H2O - SO0331 - Scharlau giá rẻ ở hcm

a) SEM image and (b) EDS spectrum obtained from NaH2PO4.H2O inhibitor.... | Download Scientific Diagram

Phase Equilibrium for the Ternary System KH2PO4 + NaH2PO4 + H2O at 303.15 K | Journal of Chemical & Engineering Data

10049-21-5, 137.99, Sodium Phosphate, Monobasic, Monohydrate, Crystal, Reagent, ACS - 6NNX9|S1395-500GM - Grainger
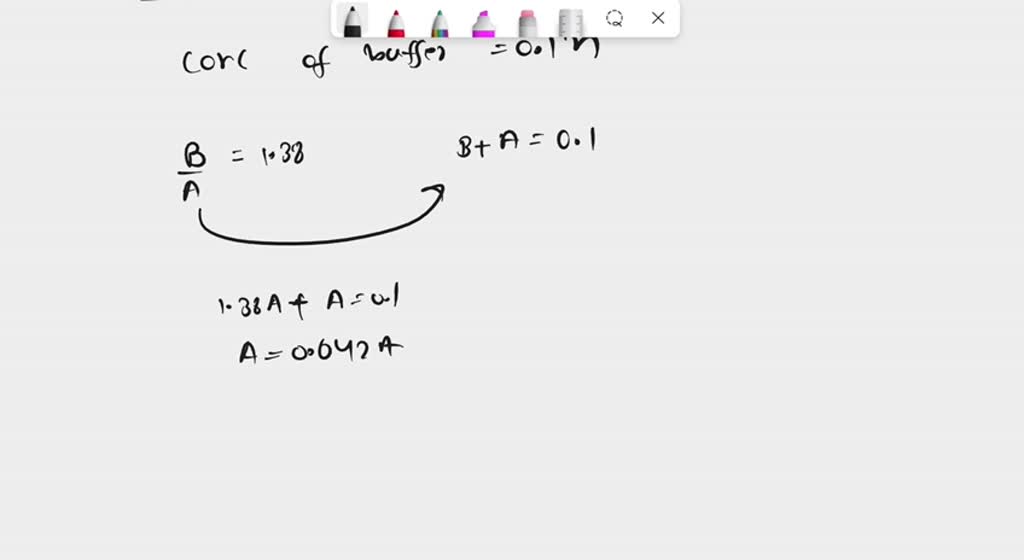
SOLVED: 1. How many g of Na2HPO4 and NaH2PO4 2H2O would you need to prepare 1L of 0.1M sodium phosphate buffer pH 7.0? (Hint= use the Henderson-Hasselbalch equation) Express your answer to
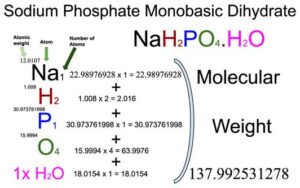
Sodium Phosphate Monobasic Monohydrate (NaH2PO4.H2O) Molecular Weight Calculation - Laboratory Notes