Cannabidiol Discovery and Synthesis—a Target‐Oriented Analysis in Drug Production Processes - Aguillón - 2021 - Chemistry – A European Journal - Wiley Online Library

Calculate the oxidation number of each sulphur atom in the following compounds. (a) Na(2)S(2)O(3) (b) Na(2)S(4)O(6) ( c) Na(2)SO(3) (d) Na(2)SO(4)

Surfactants produced from carbohydrate derivatives: A review of the biobased building blocks used in their synthesis - Ortiz - 2022 - Journal of Surfactants and Detergents - Wiley Online Library
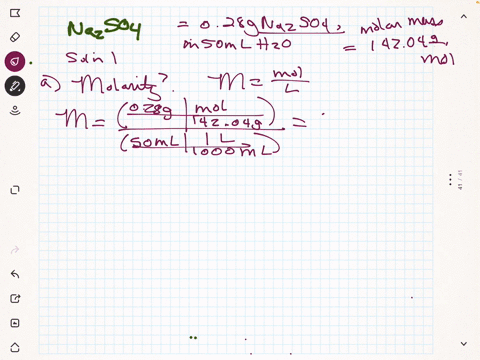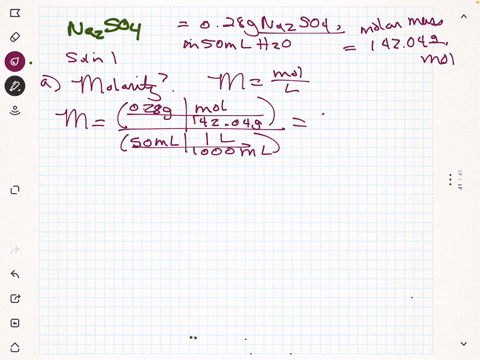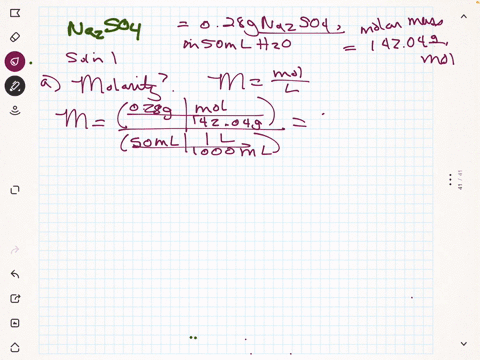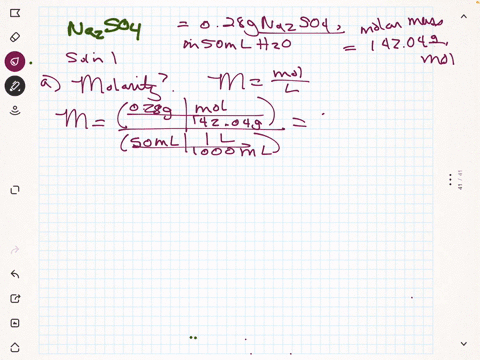
SOLVED: 1. What is the difference between Na2SO4(sodium sulfate) and Na2SO3 (sodium sulfite)? The Na2SO3 is used to convert Br2 into Br-, but the anhydrous Na2SO4 is used to dry your product.