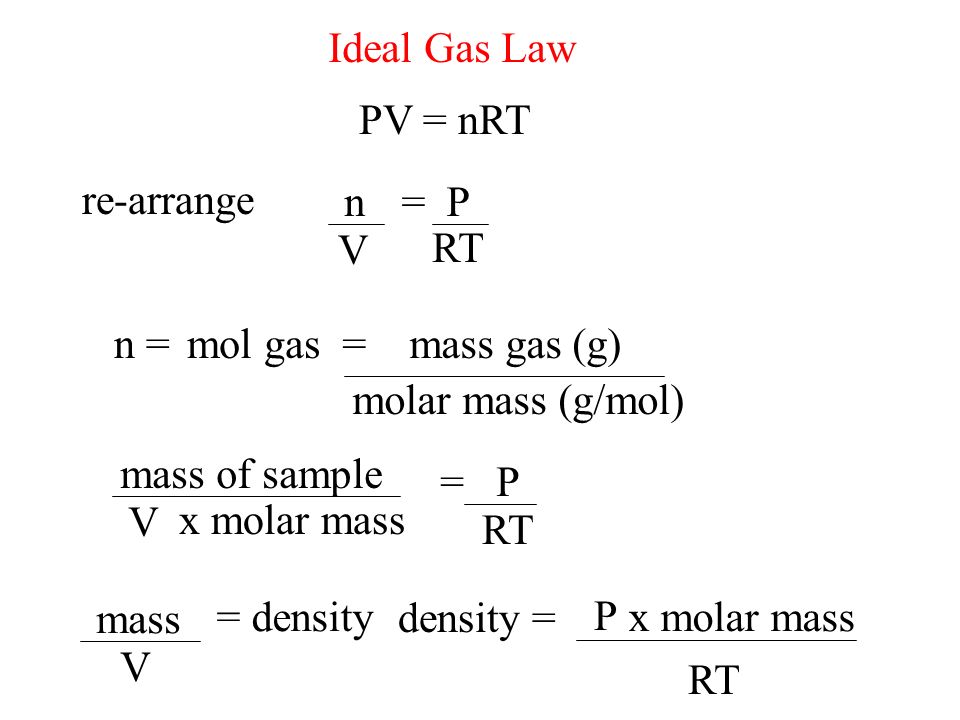
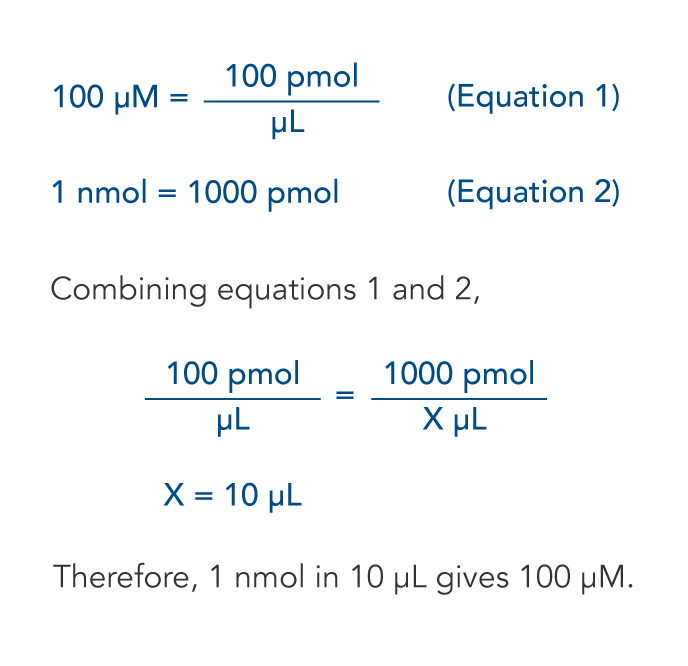
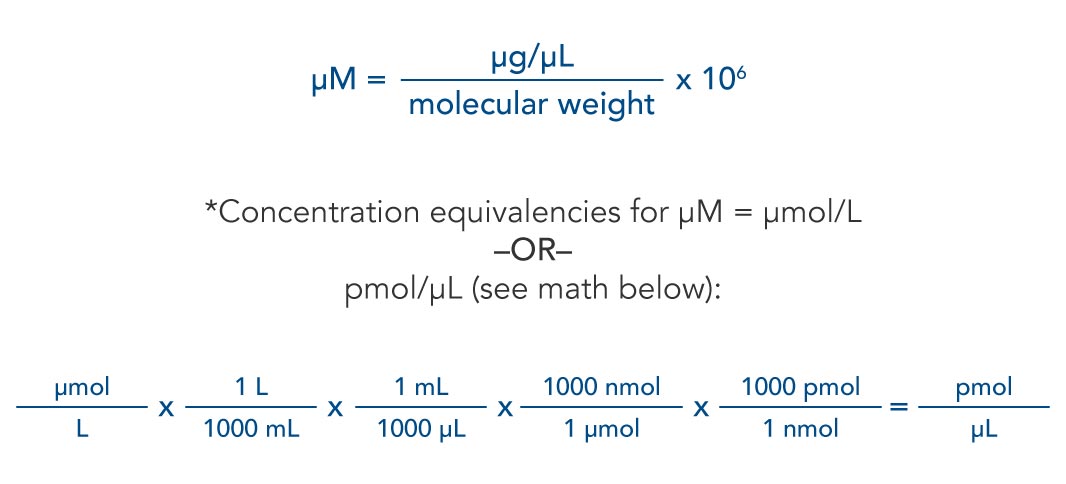
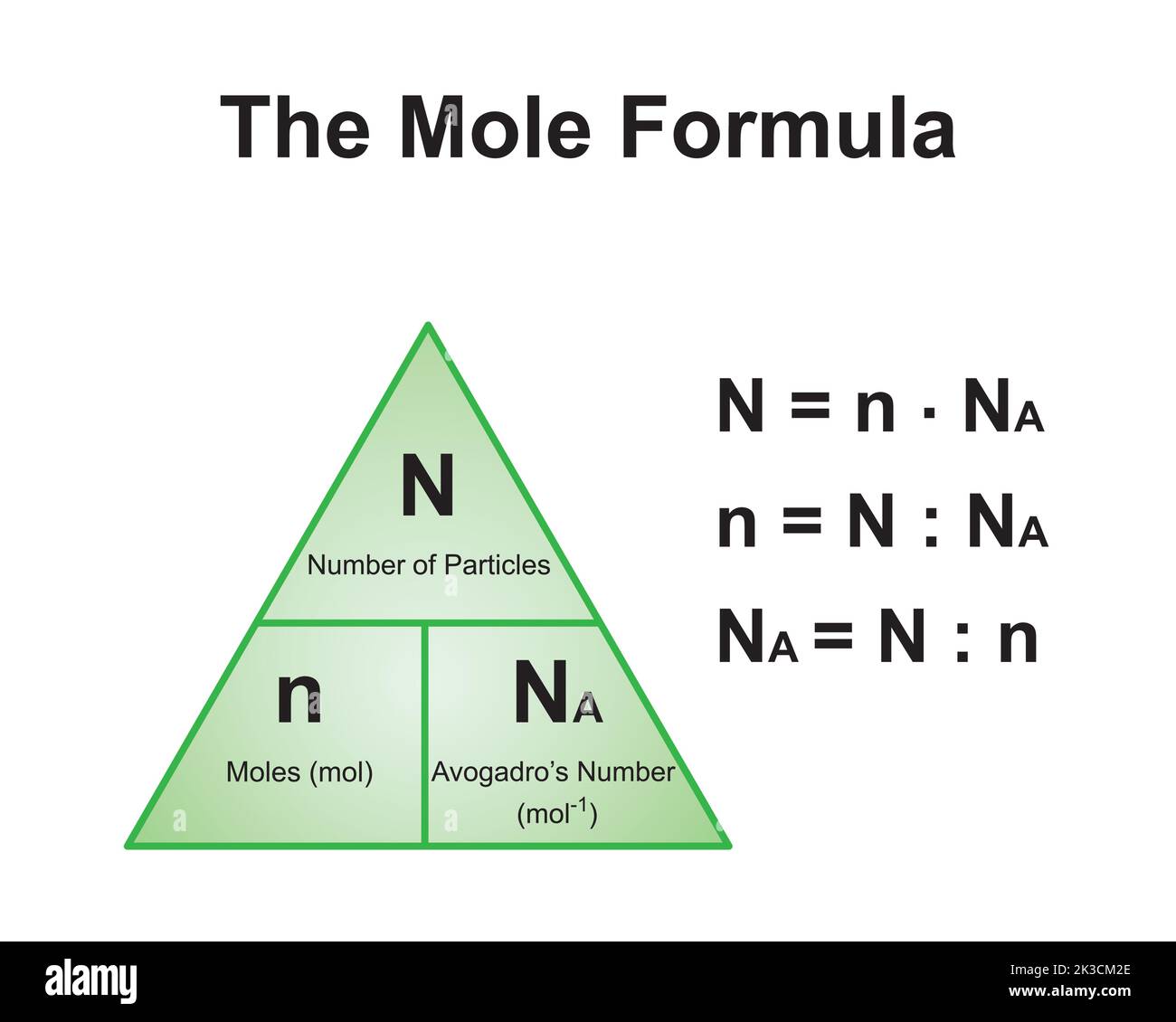
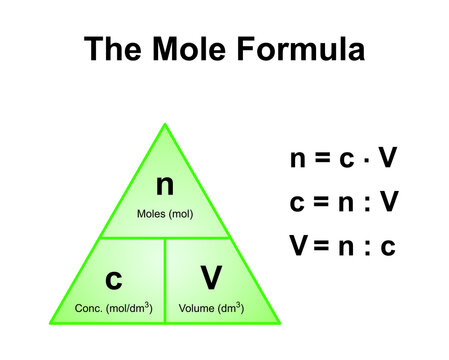
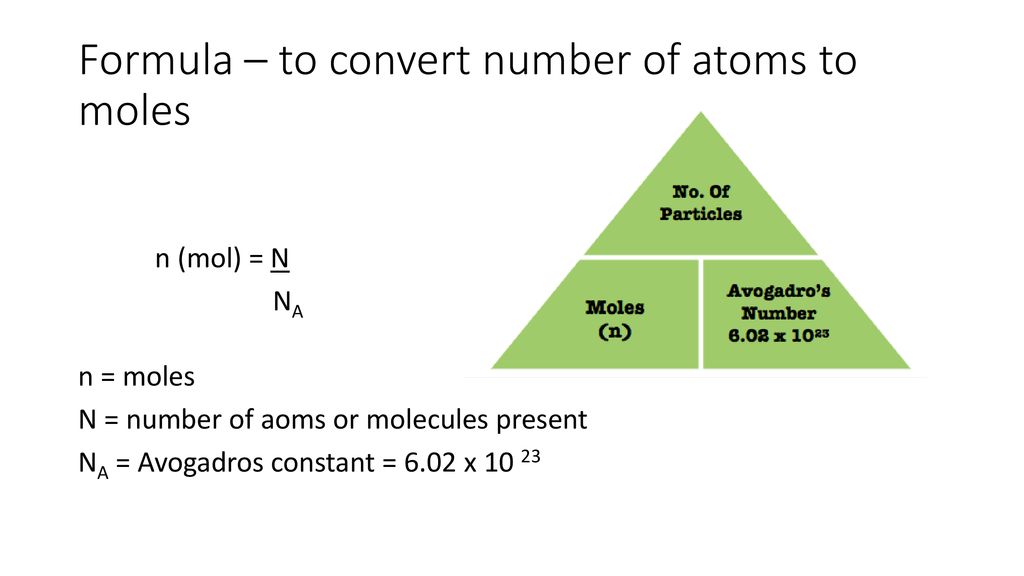
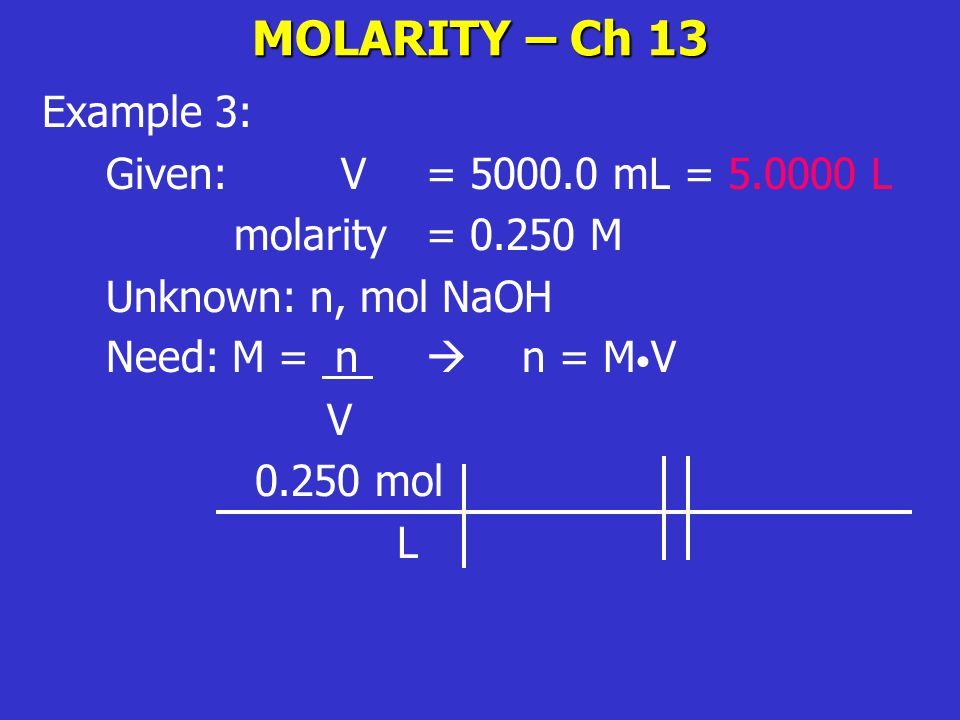
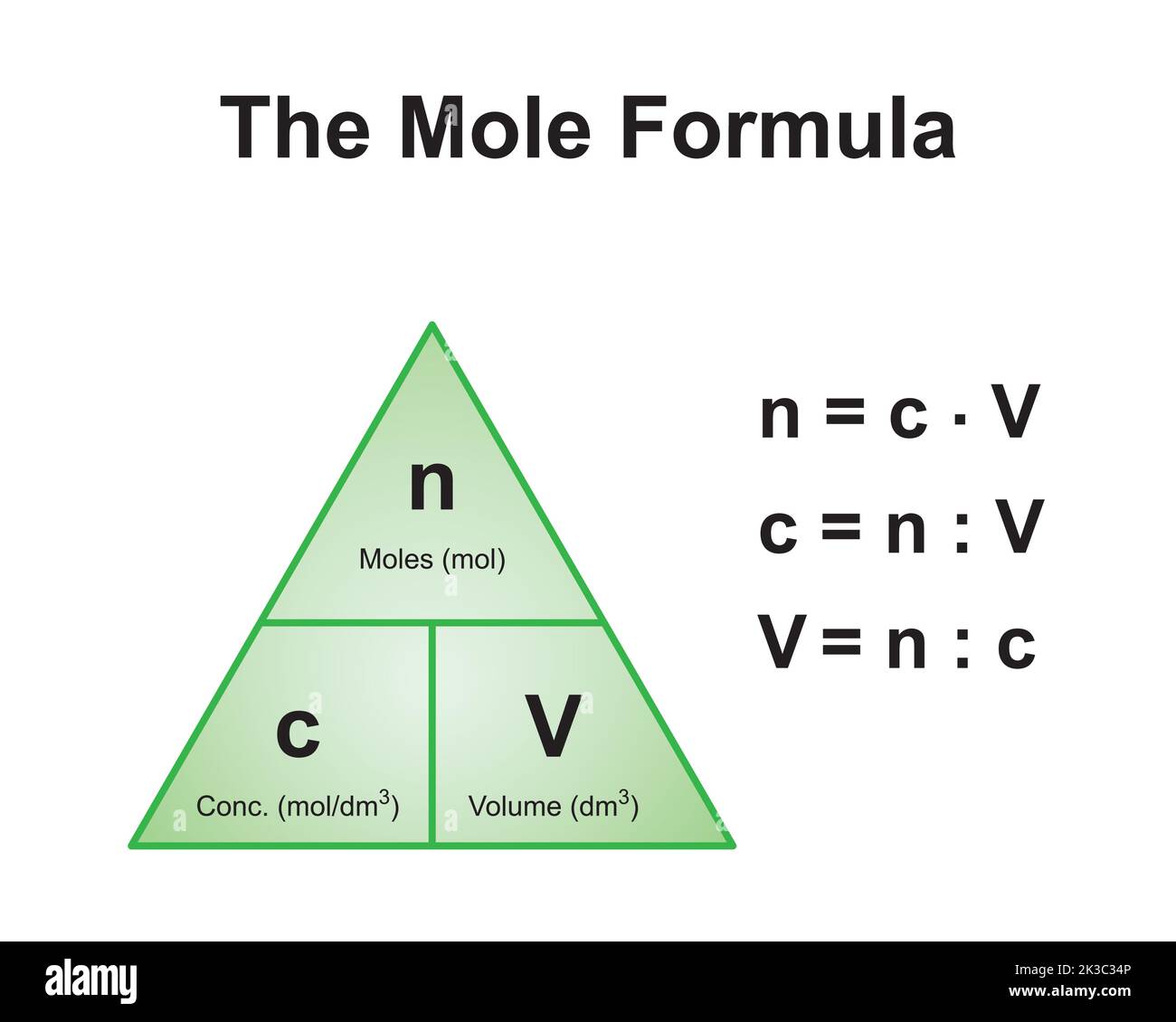
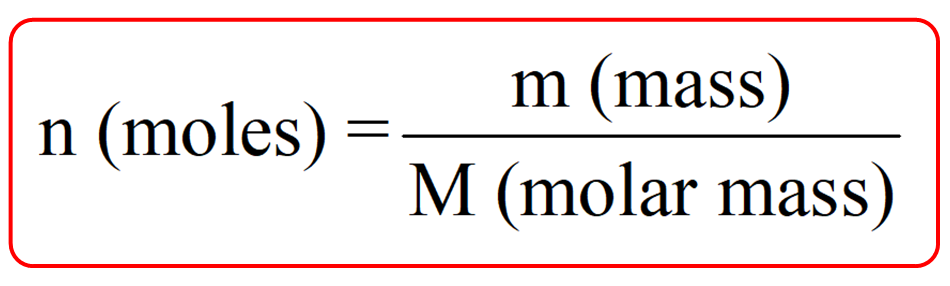The Mole And Concentration Formula Triangle Isolated On White Relationship Between Concentration Moles And Volume Cnv Stock Illustration - Download Image Now - iStock

N moles of an ideal diatomic gas are in a closed cylinder at temperature T. Suppose, on supplying heat to the gas, its temperature remains constant, but n moles get dissociated into