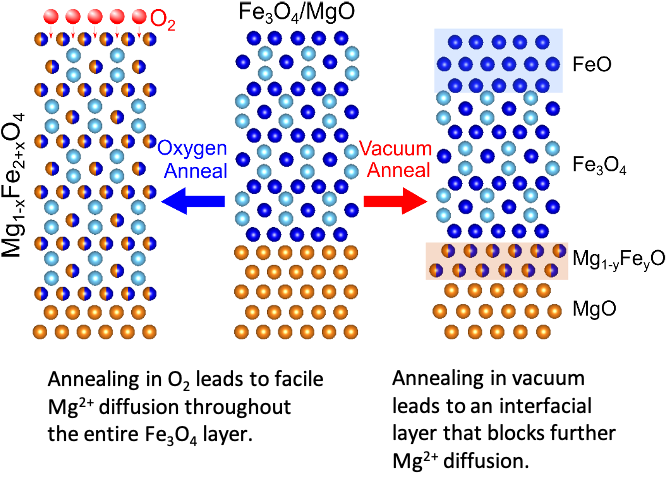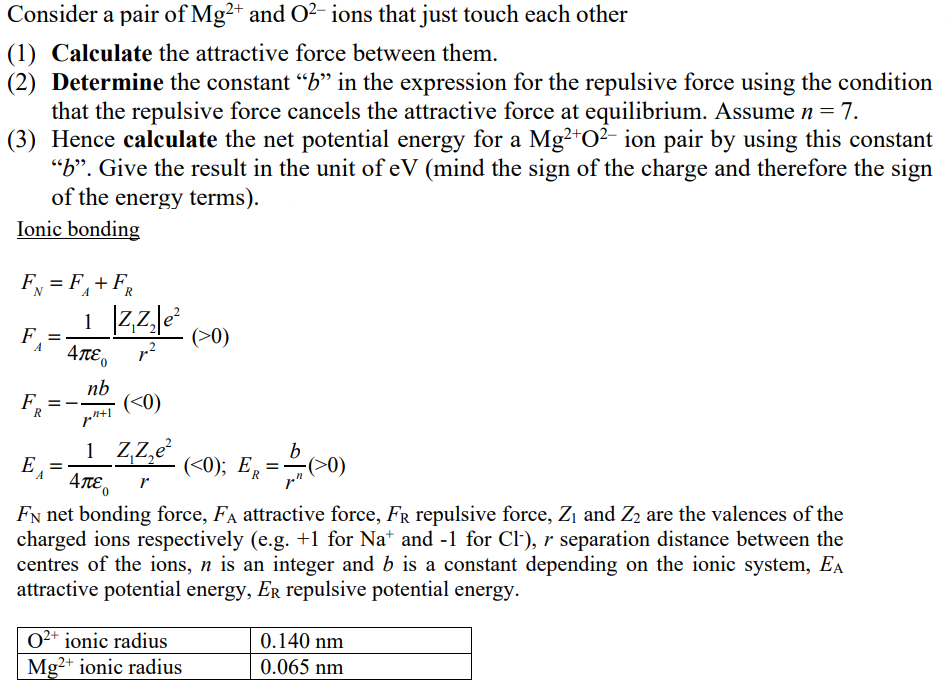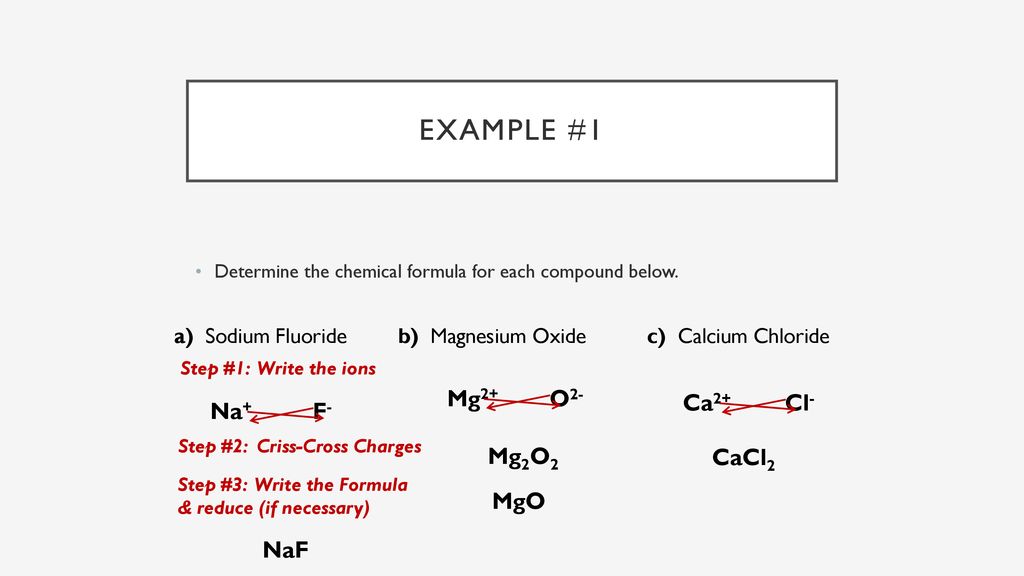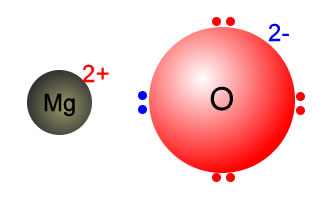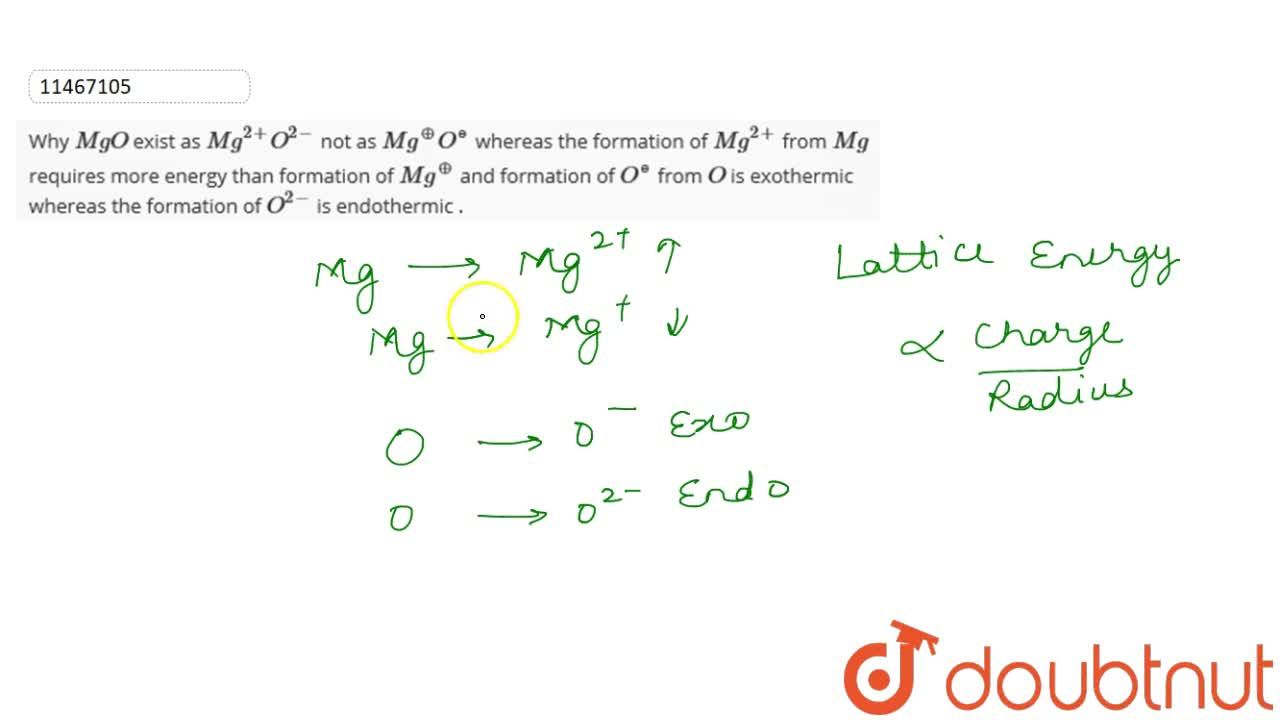
Why MgO exist as Mg^(2+)O^(2-) not as Mg^(o+)O^(ɵ) whereas the formation of Mg^(2+) from Mg requires more energy than formation of Mg^(o+) and formation of O^(ɵ) from O is exothermic whereas the
Why MgO exist as Mg^(2+)O^(2-) not as Mg^(o+)O^(ɵ) whereas the formation of Mg^(2+) from Mg requires more energy than formation of Mg^(o+) and formation of O^(ɵ) from O is exothermic whereas the

SOLVED:Consider the following energy changes: (TABLE CANNOT COPY) a. Magnesium oxide exists as Mg^2+ O^2-, not as Mg^+ O^- . Explain. b. What experiment could be done to confirm that magnesium oxide