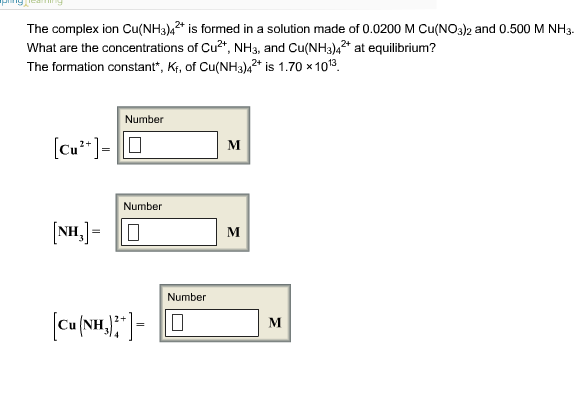
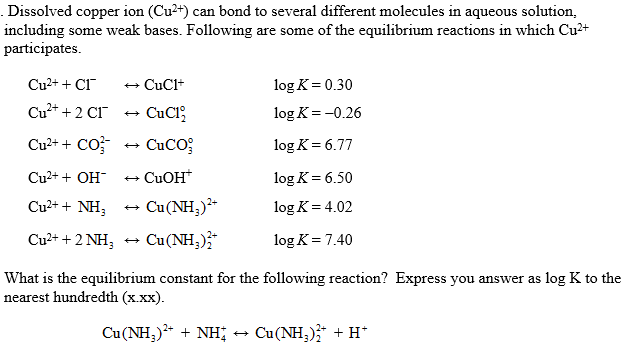
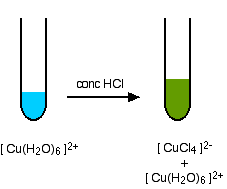
Ch 7.1 Forming Ions. Review… Cations are Groups 1A, 2A, and 3A They have positive charges. Anions are Groups 5A, 6A, and 7A They have negative charges. - ppt download

Copper Cu transition metal Chemistry copper(I) Cu+ copper(II) Cu2+ ion complex ions ligand substitution compounds redox chemical reactions principal oxidation states +1 +2 GCE AS A2 IB A level inorganic chemistry revision


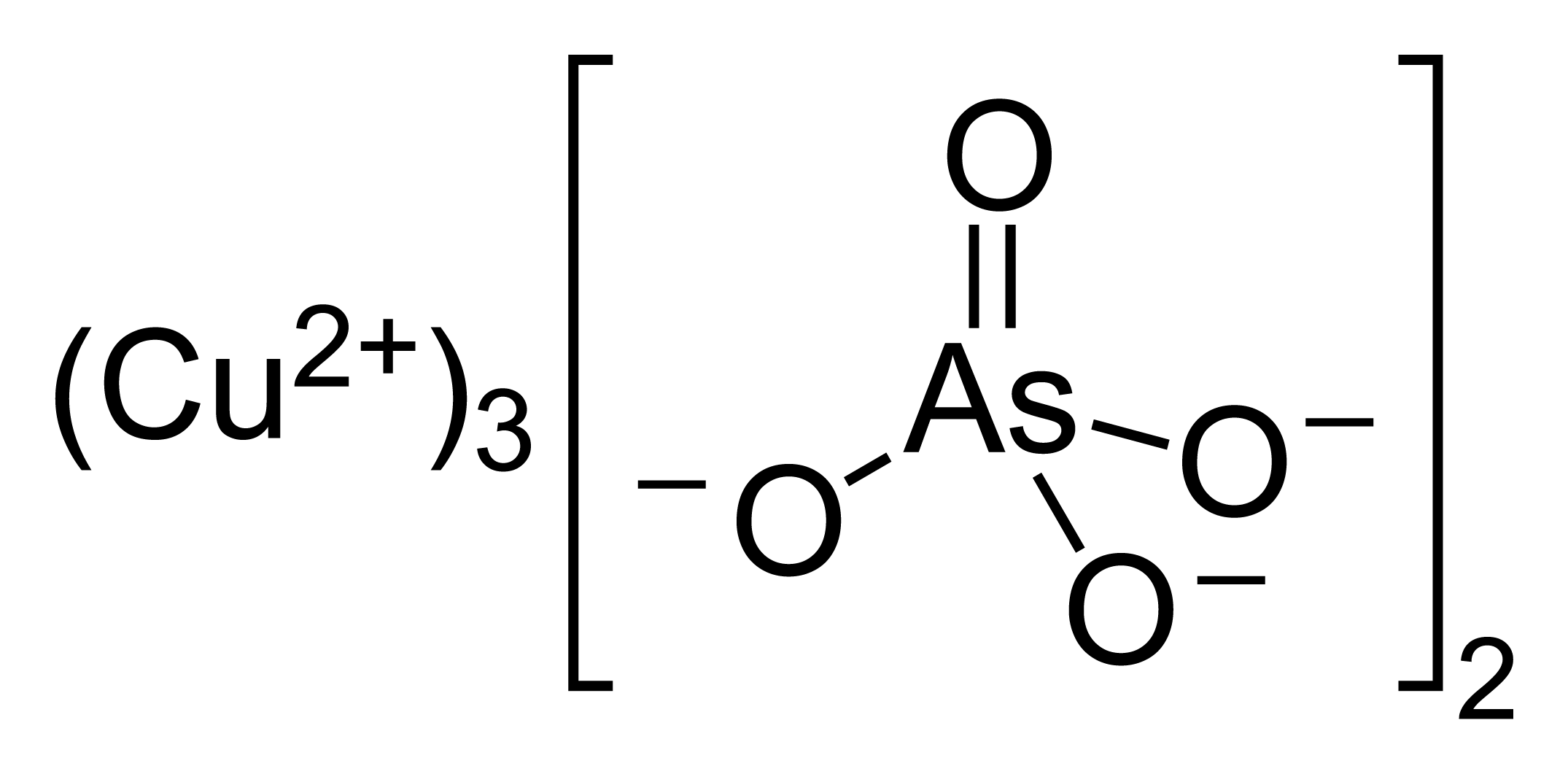
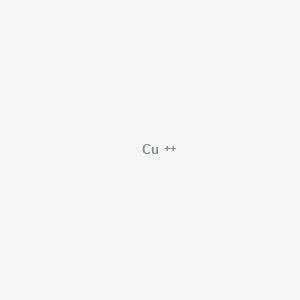

![PDF] Removal of copper ions Cu (II) from industrial wastewater | Semantic Scholar PDF] Removal of copper ions Cu (II) from industrial wastewater | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ddffa5eb8683308f5473a9ab8f43aa78832748fe/3-Figure1-1.png)