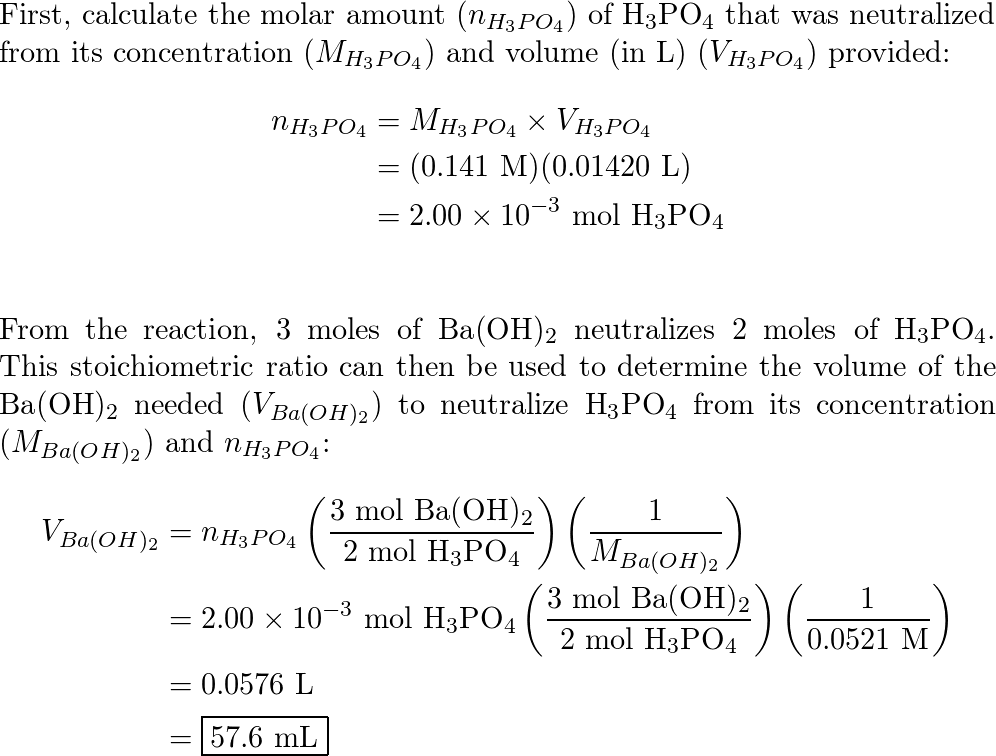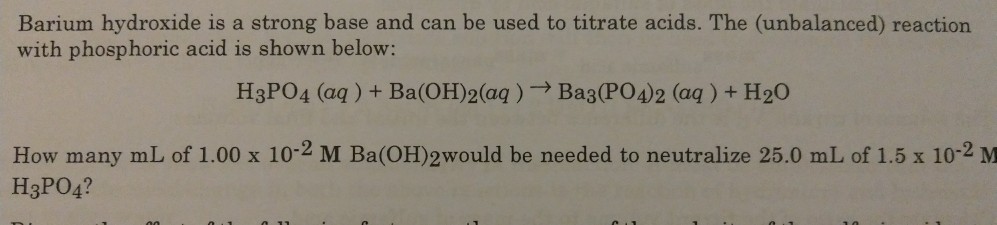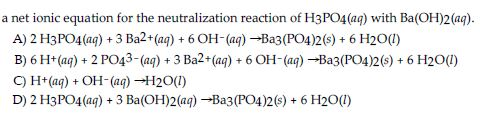
The volume of 3 M Ba(OH)^(2) solution required to neutralize completely 120 mL of 1.5M H(3)PO(4) solution is :
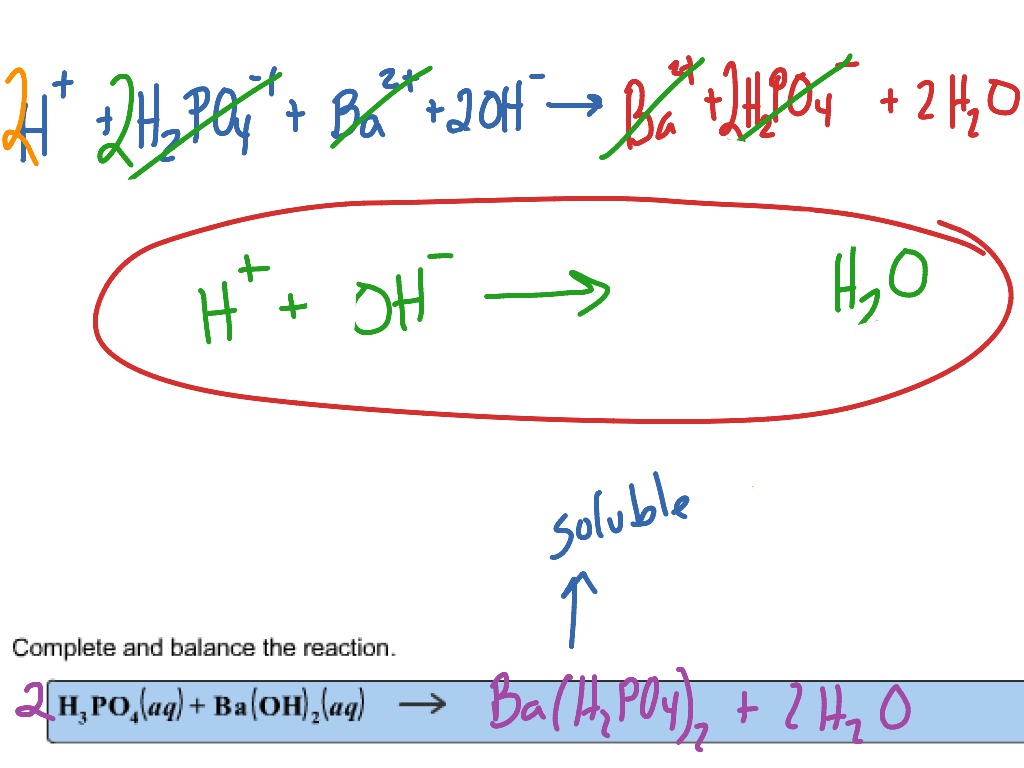
SOLVED: According to the balanced chemical equation 2 H3PO4(aq) Ba(OH) (aq) Ba3(PO4)2(5) Hzo() Express this equation With microscopic and macroscopic point of view: micfoscopic: macroscopic:
✓ Solved: What volume of 0.0521 M Ba(OH) 2 is required to neutralize exactly 14.20 mL of 0.141 M H 3...
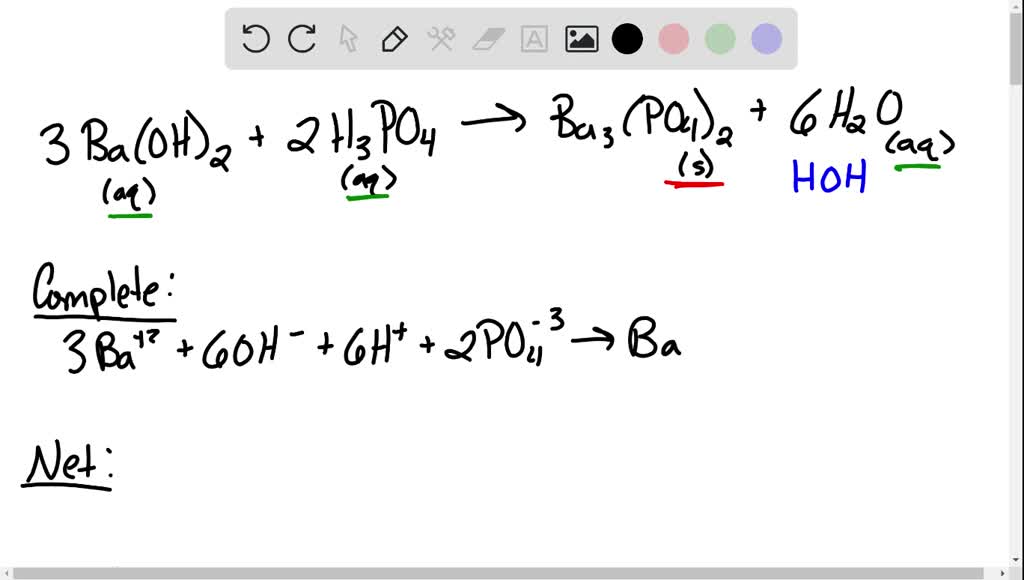
SOLVED: What would be the total ionic equation for 3Ba(OH)2 (aq) + 2H3PO4 (aq) → Ba3(PO4)2 (s) + 6H2O (aq)? Identify the spectator ions of this reaction.
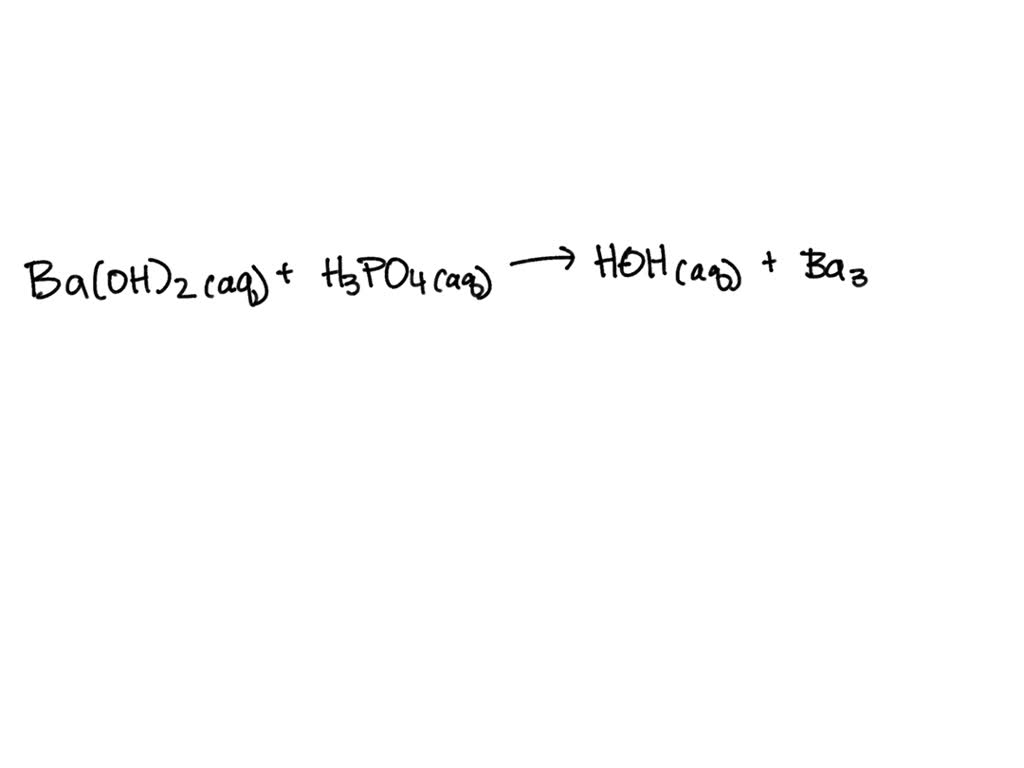
SOLVED: When a solution containing barium hydroxide is added to a solution containing phosphoric acid (HaPO4), a white precipitate forms and settles to the bottom of the beaker: What is the white
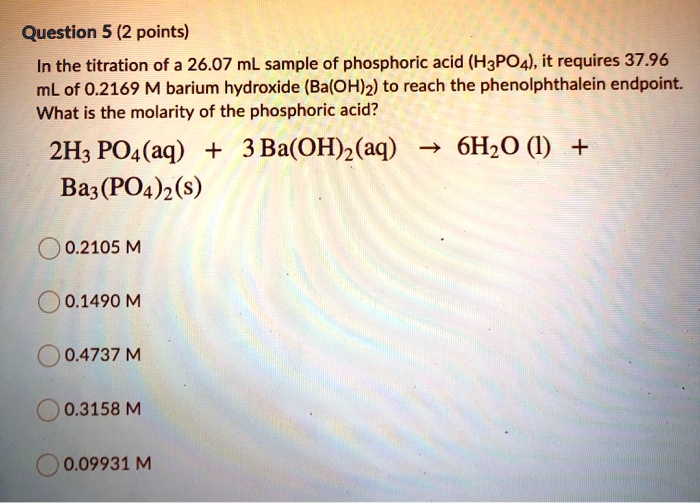
SOLVED: Question 5 (2 points) In the titration of a 26.07 mL sample of phosphoric acid (H3POA), it requires 37.96 mL of 0.2169 M barium hydroxide (Ba(OH)2) to reach the phenolphthalein endpoint: