The System CaCl2–H2O: Thermodynamic Modeling and Flow Calorimetry Experiments at Elevated Temperatures and Pressures | Journal of Chemical & Engineering Data
![Calculate the enthalpy change of freezing of 1.0 mol of water at 10^(@)C to ice at -10^(@)C, Delta(fus)H=6.03 kJ mol^(-1) at 0^(@)C. C(P)[H(2)O(l)]=75.3 J mol^(-1) K^(-1) C(P)[H(2)O(s)]=36.8 J mol^(-1) K^(-1) Calculate the enthalpy change of freezing of 1.0 mol of water at 10^(@)C to ice at -10^(@)C, Delta(fus)H=6.03 kJ mol^(-1) at 0^(@)C. C(P)[H(2)O(l)]=75.3 J mol^(-1) K^(-1) C(P)[H(2)O(s)]=36.8 J mol^(-1) K^(-1)](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/34965050_web.png)
Calculate the enthalpy change of freezing of 1.0 mol of water at 10^(@)C to ice at -10^(@)C, Delta(fus)H=6.03 kJ mol^(-1) at 0^(@)C. C(P)[H(2)O(l)]=75.3 J mol^(-1) K^(-1) C(P)[H(2)O(s)]=36.8 J mol^(-1) K^(-1)
![Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer–Villiger monooxygenases - Chemical Communications (RSC Publishing) Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer–Villiger monooxygenases - Chemical Communications (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/B504921K)
Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer–Villiger monooxygenases - Chemical Communications (RSC Publishing)
![PDF] Energetics of Large Water Clusters [up to (H2O)20] by means of Explicitly Correlated, Localized Coupled Cluster Methods | Semantic Scholar PDF] Energetics of Large Water Clusters [up to (H2O)20] by means of Explicitly Correlated, Localized Coupled Cluster Methods | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/2138338cc552e5f630bfd9ff0acdfc12e059e663/6-Table1-1.png)
PDF] Energetics of Large Water Clusters [up to (H2O)20] by means of Explicitly Correlated, Localized Coupled Cluster Methods | Semantic Scholar

SOLVED: Use these constants to answer the following questions Cpice 2.06 Jlg %C Cpsteam Jig"C AHtus 334 Jlg AHvap 2.02 Jlg'C Cp water 4.18 2260 Jlg 4.80 kg of ice at 0PC

SOLVED: When 1.053 g of tartaric acid (C4H6O6(s), MM = 150.1 g∙mol−1), is burned in a bomb calorimeter (Cbomb = 878 J∙ oC−1) that contains 968.6 g of water (Cp (H2O) =

The enthalpy change for a reaction at equilibrium is - 20.5 kJ mol ^-1 . Then the entropy change for this equilibrium at 410 K is:



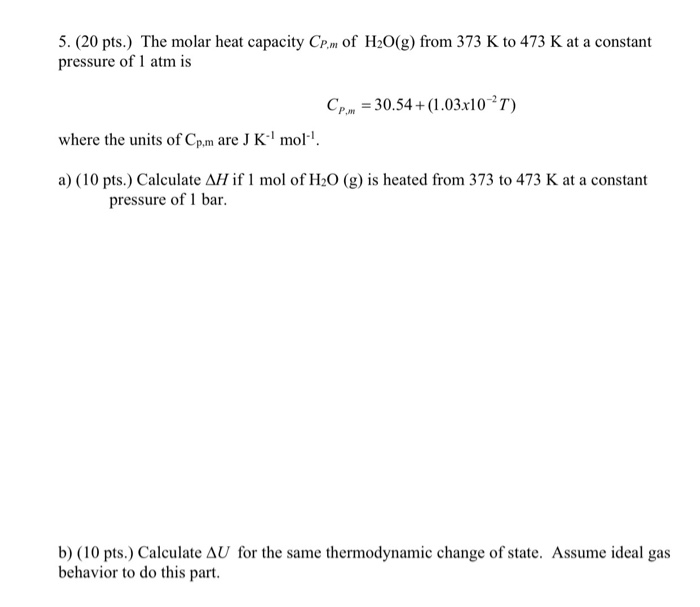
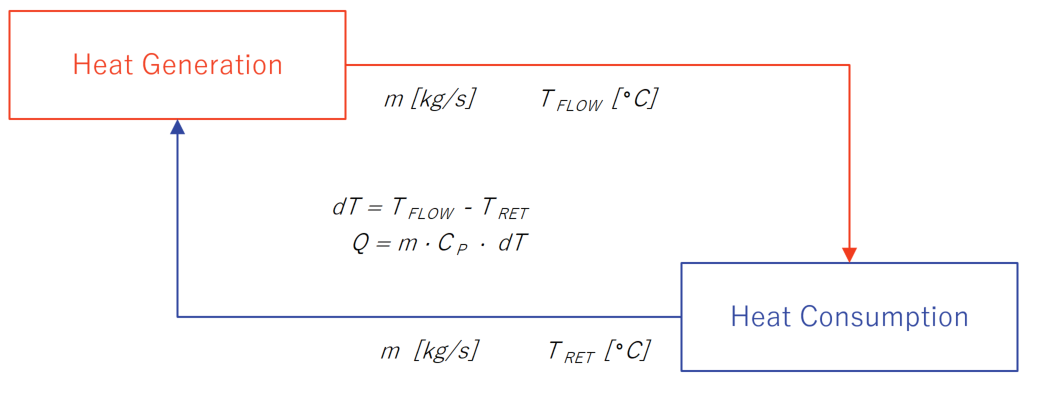

![Solved Heat Capacities pIH20l 37.65 J/K-mol Cp[H2O(l)]-75.29 | Chegg.com Solved Heat Capacities pIH20l 37.65 J/K-mol Cp[H2O(l)]-75.29 | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F9c6%2F9c6322fb-d755-40a1-8987-9484af98164f%2FphpxcNGUz.png)