Amazon.com : Pure Original Ingredients Calcium Sulfate (5 lb) Baking, Water Treatment & Gardening : Patio, Lawn & Garden

Influence of the Process Parameters on the Formation of CaSO4·0.5H2O Whiskers – topic of research paper in Nano-technology. Download scholarly article PDF and read for free on CyberLeninka open science hub.

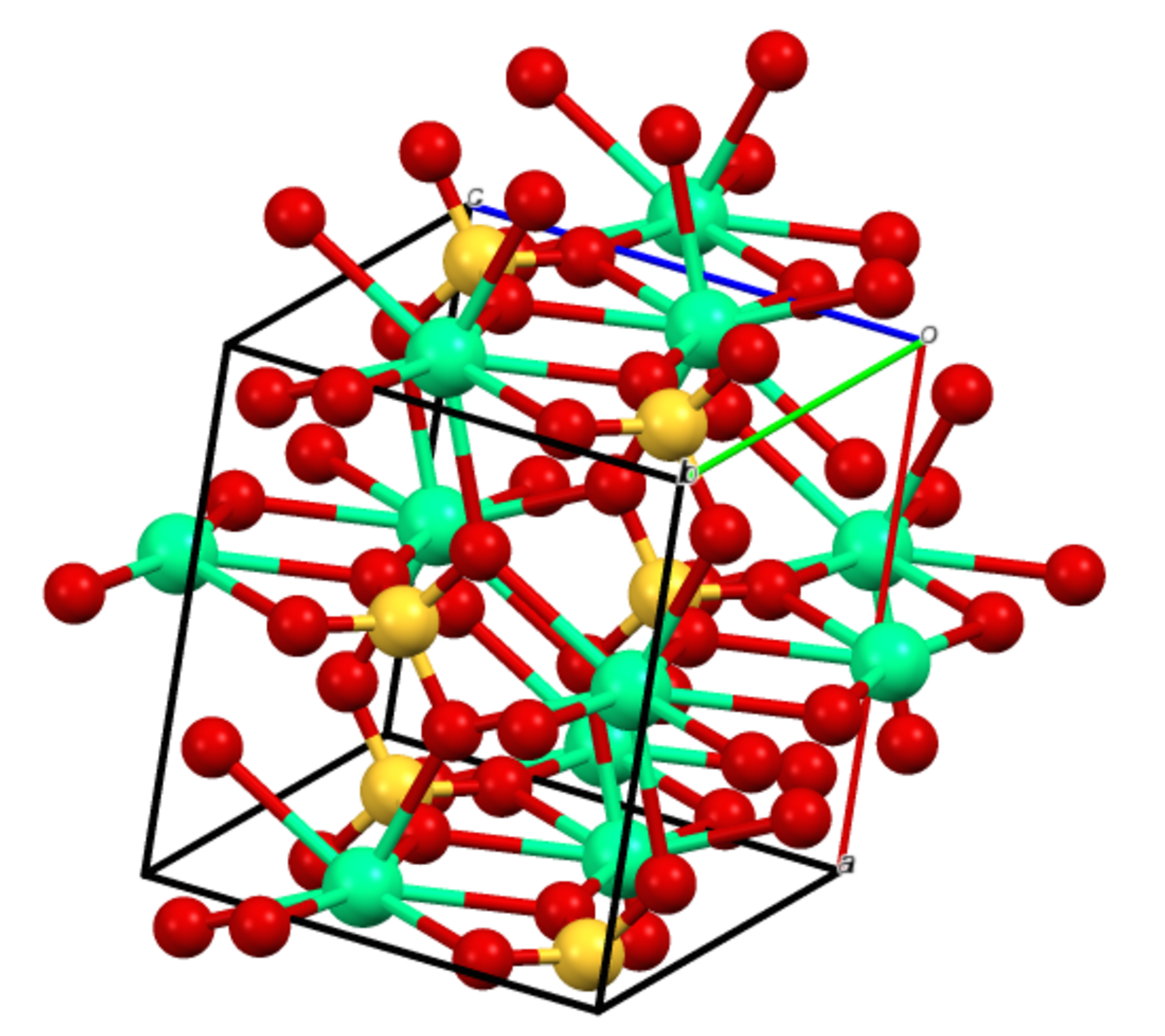
Crystal structure of (a) γ‐CaSO4 and (b) bassanite CaSO4 ⋅ 0.5H2O, as... | Download Scientific Diagram
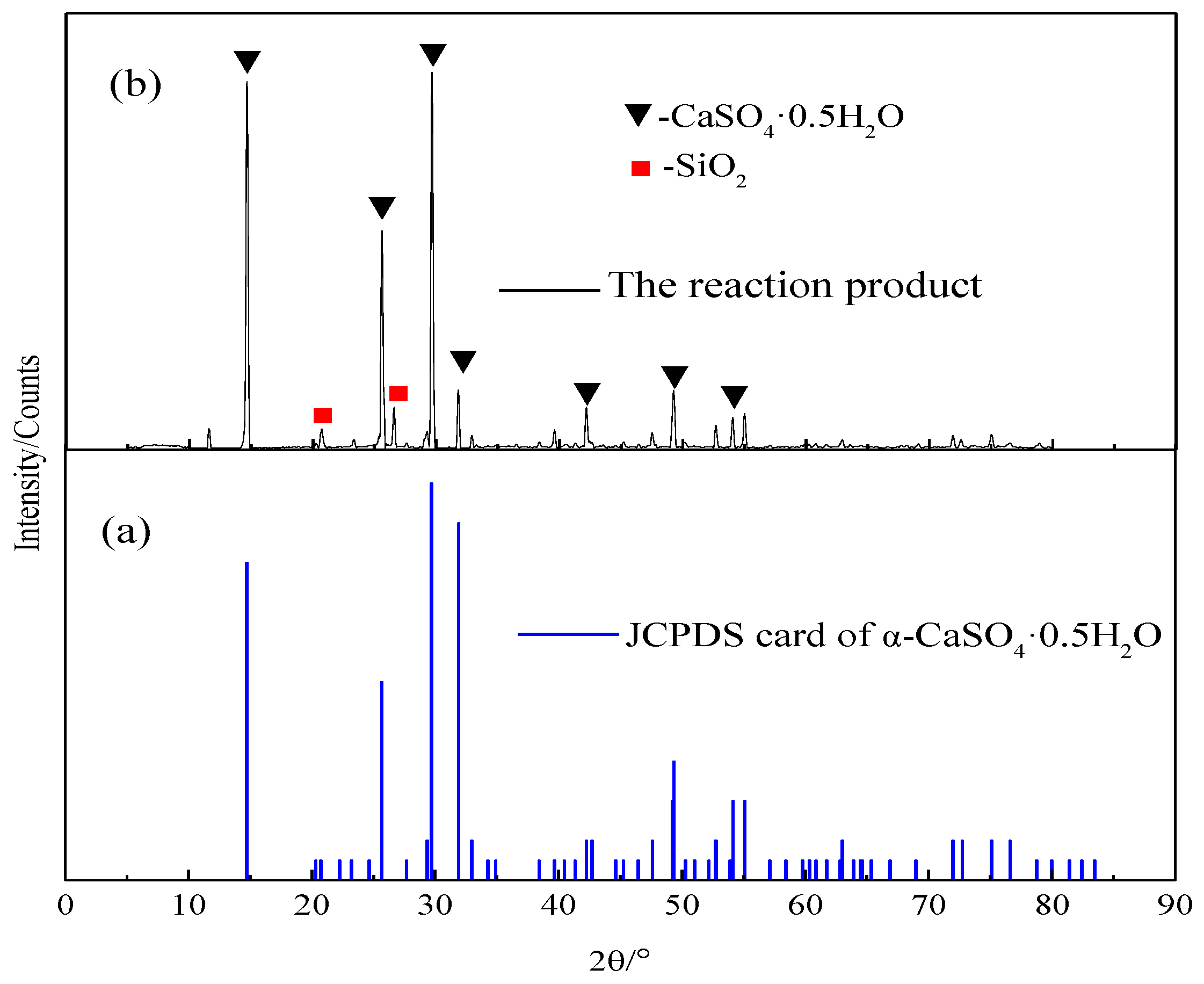
Crystals | Free Full-Text | Effect of Molecular Structure of Organic Acids on the Crystal Habit of α-CaSO4·0.5H2O from Phosphogypsum
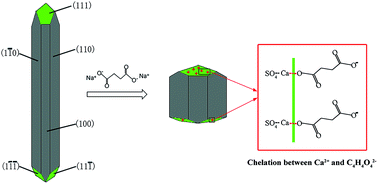
Synthesis of α-CaSO4·0.5H2O from flue gas desulfurization gypsum regulated by C4H4O4Na2·6H2O and NaCl in glycerol-water solution - RSC Advances (RSC Publishing)

Direct synthesis of single-phase α-CaSO4·0.5H2O whiskers from waste nitrate solution - ScienceDirect
Force field for calcium sulfate minerals to predict structural, hydration, and interfacial properties
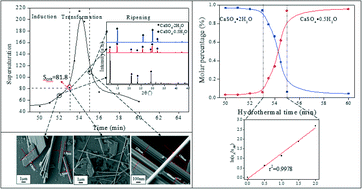
Supersaturation-induced hydrothermal formation of α-CaSO4·0.5H2O whiskers - CrystEngComm (RSC Publishing)
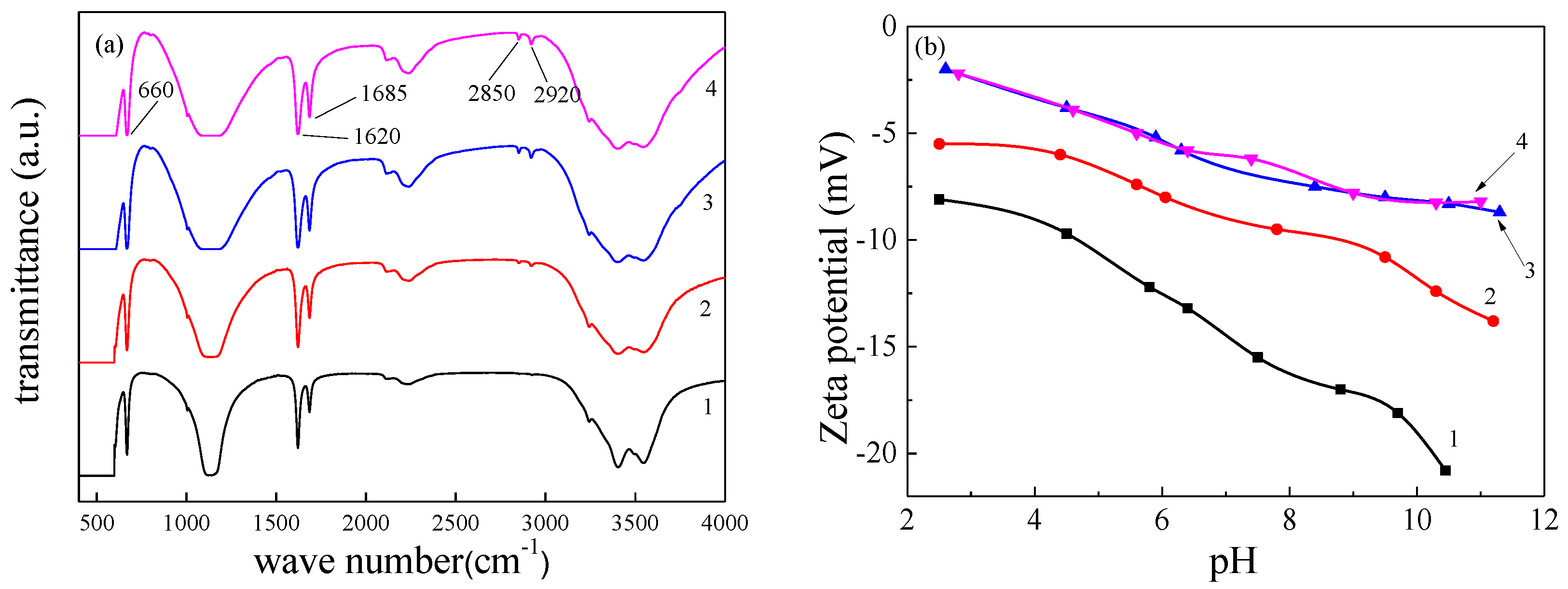
Crystals | Free Full-Text | Influence of Alkyl Trimethyl Ammonium Bromides on Hydrothermal Formation of α-CaSO4·0.5H2O Whiskers with High Aspect Ratios
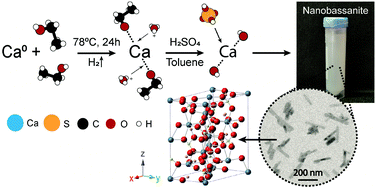
Synthesis of high surface area CaSO4·0.5H2O nanorods using calcium ethoxide as precursor - Chemical Communications (RSC Publishing)
![PDF] Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Semantic Scholar PDF] Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7cebe8c890fa31a7af18365a4240a254e5398399/2-Table1-1.png)