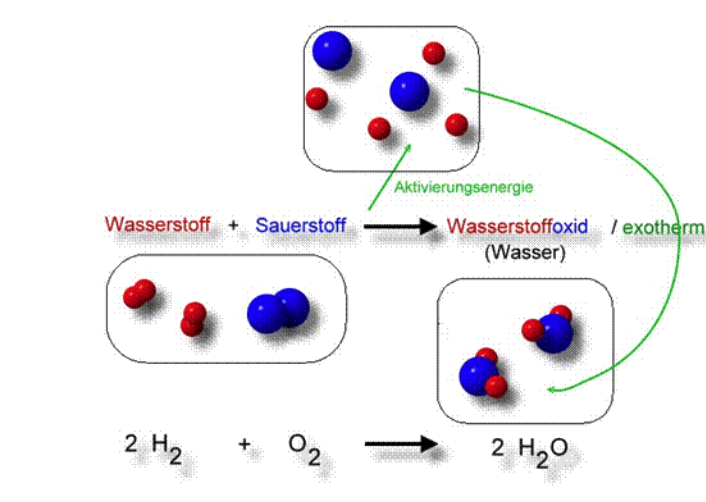
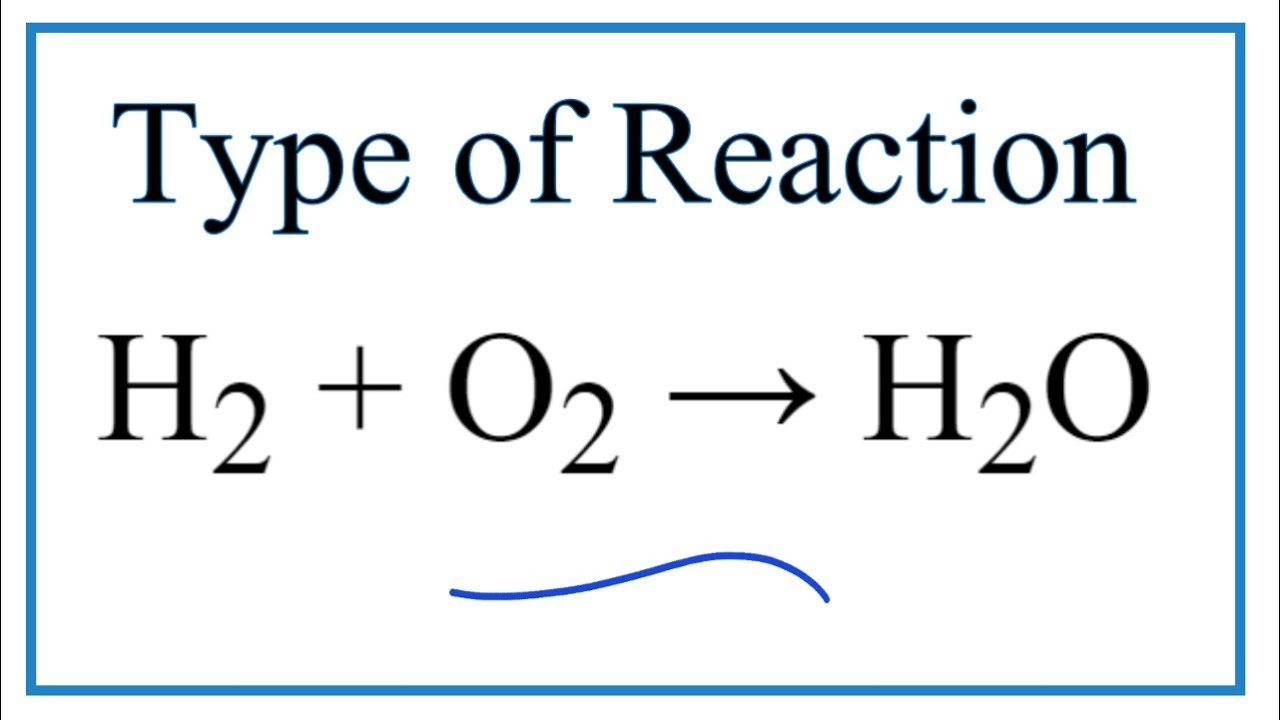Synthesis of cis-Cu(gly)2·H2O, trans-Cu(gly)2, and cis-Ni(gly)2(H2O)2 and their Characterization Using Thermal and Spectroscopic Techniques—A Capstone Inorganic Laboratory | Journal of Chemical Education
![The crystal structure of trans potassium dioxalatodiaquochromiate, K[CR(C2O4)2(H2O)2].3H2O - Van Niekerk - 1951 - Acta Crystallographica - Wiley Online Library The crystal structure of trans potassium dioxalatodiaquochromiate, K[CR(C2O4)2(H2O)2].3H2O - Van Niekerk - 1951 - Acta Crystallographica - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/29dbd8f8-5ed2-49f2-94d2-4332b7d55fcc/s0365110x51000064.fp.png)
The crystal structure of trans potassium dioxalatodiaquochromiate, K[CR(C2O4)2(H2O)2].3H2O - Van Niekerk - 1951 - Acta Crystallographica - Wiley Online Library
5. If 2 moles ofSF4 is mixed with 18g of H2O then incorrect statement is 1 only 0.5 mole of SF4 is reacted 2 H2O is limiting reagent 3 2 moles of
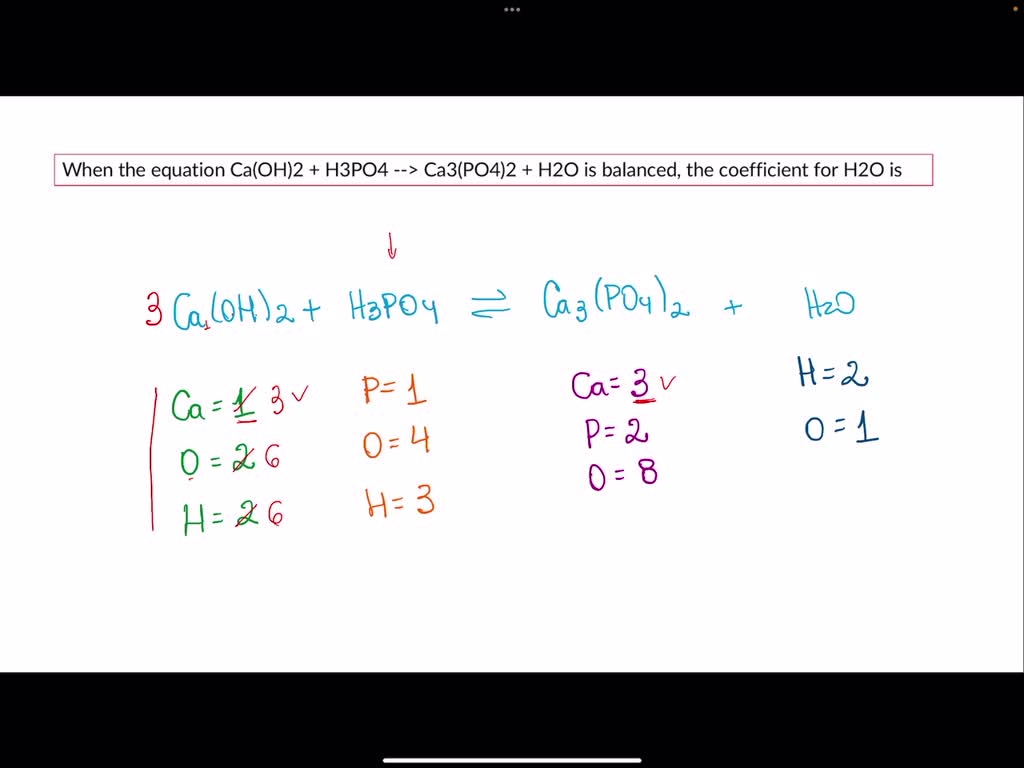
SOLVED: When the equation Ca(OH)2 + H3PO4 –> Ca3(PO4)2 + H2O is balanced, the coefficient for H2O is

Complete the following equation: Na2O2 + 2H2O → (W) + H2O2 2KO2 + 2H2O→ (X) + (Y) + O2 Na2O + CO2→ (Z)

Overall reaction of Cathode and Anode: Cathode: 2 H2O(l) + 2e− → H2(g)... | Download Scientific Diagram
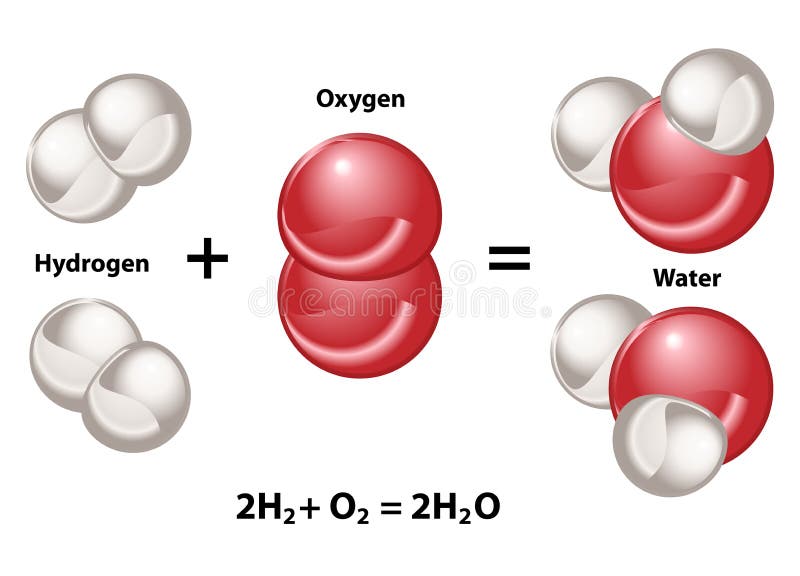
Molecular Compound in H2O Water Molecule Stock Vector - Illustration of creating, compounds: 195911340

Chemical Structure H2o And 2h2o Stock Photo - Download Image Now - 2015, Chemical Formula, Chemistry - iStock


![Solved [Cl-] low: Oxidation: 2 H2O 02 + 4H+ 4e Reduction: 2 | Chegg.com Solved [Cl-] low: Oxidation: 2 H2O 02 + 4H+ 4e Reduction: 2 | Chegg.com](https://media.cheggcdn.com/media/ac0/ac051806-14e4-4144-a9a5-c6edb1f2a071/php07e7Pp)